by Hanumantha Rao Vutukuri, h.r.vutukuri@utwente.nl, ETH Zürich, Switzerland (currently: University of Twente, the Netherlands) and Dmitry A. Fedosov, d.fedosov@fz-juelich.de Forschungszentrum Jülich, Germany
Towards the Minimalistic Design of Life-like Abiotic Systems
8th March 2022
How Active Biopolymer Networks Shape the Cell Membrane
11th March 2022
Towards the Minimalistic Design of Life-like Abiotic Systems
8th March 2022
How Active Biopolymer Networks Shape the Cell Membrane
11th March 2022

Biological cells are fascinating soft microsystems, which can process chemical and mechanical information and actively respond to internal and external perturbations [1]. One of the important fundamental challenges of modern science is to understand cellular self-organization and function, and reconstitute the basic principles of life. To this end, engineering simple cell-mimicking systems allows us not only to learn about their natural counterparts, but also to derive design principles of soft functional micro-robots capable of performing cell-like and beyond-nature tasks. Here, we highlight our recent collaborative study [2] between the ETH Zürich, Switzerland, and the Forschungszentrum Jülich, Germany, on a novel active system of self-propelled particles (SPPs) enclosed in a lipid vesicle, which exhibits dramatic shape changes resembling those of biological cells.
Artificial soft-matter systems, such as giant unilamellar vesicles, have been successfully used as a minimal model system to mimic biological cells [3]. Despite numerous studies on the design of complex vesicle systems, the realization of a minimal system, in which localized active forces can strongly deform lipid membranes from the inside and induce dramatic shape changes, remains challenging.
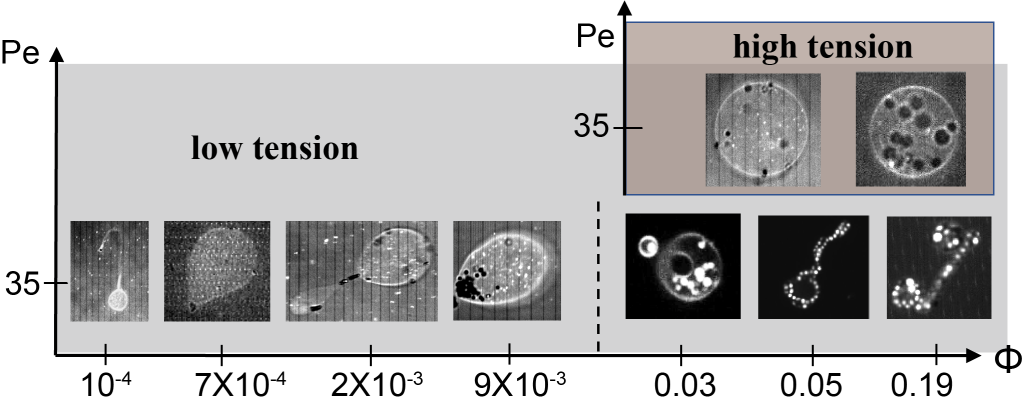
We have addressed this challenge through the development of a unique synthetic system where active particles are confined within giant vesicles [2]. In this system, the cell membrane is mimicked by a dioleoylphosphatidylcholine (DOPC) lipid bilayer, and localized forces are generated by self-propelled Janus particles catalytically driven in the presence of hydrogen peroxide H2O2. In experiments, the volume fraction (Φ) of active particles was modulated to control overall activity strength as well as the tension of the membrane to alter membrane properties. In order to understand the emergent behaviour of this active system, we have also performed Langevin dynamics simulations of active Brownian particles enclosed in thin membrane shells, described by dynamically triangulated surfaces.
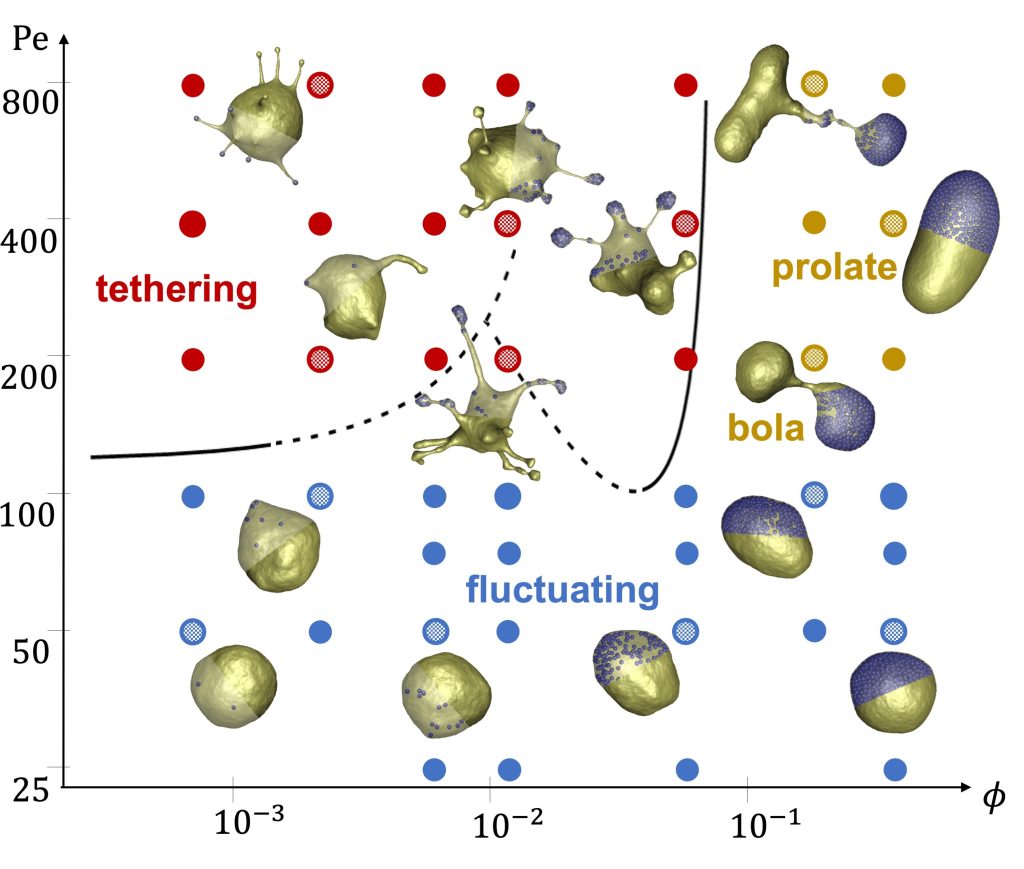
Our combined experimental and simulation study reveals the existence of a plethora of novel vesicle shapes, which do not exist in equilibrium systems. Figures 1 and 2 show the formation of tether-like protrusions and highly branched, dendritic structures at low and moderate particle loadings. At high volume fractions of active particles, globally deformed vesicle shapes such as bola shapes are generally observed, resulting even in vesicle division in some cases. Note that at low Φ, tether formation is initiated by single SPPs, while at moderate volume fractions, the initiation of tethers is performed by small particle clusters. The clustering of active particles is enhanced in places with a high local membrane curvature, such that there exists a feedback mechanism between SPP accumulation and local curvature induction by particle clusters. The transition to tethering as a function of the Peclet number (Pe, characterizes particle propulsion strength) is also well captured by a theoretical model, which is indicated by the black lines in Figure 1. The suppression of membrane tethers at large Φ is due to the induction of significant active tension mediated by the collective behaviour of SPPs. Finally, experimental and simulation results correspond well to each other, suggesting that the simulation model properly captures the physical principles of this active system.
Strikingly, ‘less is more’ for this active system, such that a moderate number of SPPs or a moderate total strength of active forces induce the most dramatic vesicle shape changes. This is also likely the case for biological cells, where the cytoskeleton actively exerts local forces to dynamically sculpt the cell shape.
Our study provides a foundation for future developments of cell-mimicking artificial systems, such as small-scale soft robots and synthetic cells.
References
[1] D. A. Fletcher and R. D. Mullins, Nature 463, 485 (2010).
[2] H. R. Vutukuri et al., Nature 586, 52 (2020).
[3] R. Dimova and C. Marques, The Giant Vesicle Book (CRC Press, 2019).